Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


Craig Voellmicke, Vice President, Business Development for CSP Technologies, Inc. – a leader in packaging solutions that ensure product protection, enhance brand recognition and improve consumer experiences – will headline a session concerning pharmaceutical compliance at the IPA Annual Stability Program Conference, May 31–June 2 in Montreal.
Titled “Enhancing Shelf Life and Simplifying Packaging for Transdermal & Oral Solid Dose Drugs with Engineered Polymers for Head-Space Management,” Mr. Voellmicke’s presentation will showcase CSP Technologies’ patented approach to tackling headspace challenges, including moisture and oxygen ingress, whose negative impacts can be detrimental to a pharmaceutical product’s efficacy.
Despite being two very different drug delivery platforms, oral solid doses and transdermal patches can face similar packaging headspace challenges that impact shelf life; besides moisture and oxygen ingress, exposure to certain conditions can lead to the formation of volatile organic compounds (VOCs) and other gases that, in turn, may interact with drug products and capsules, adversely impacting their stability.
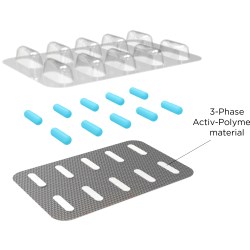
When utilized within pouch, blister and bottle packaging, CSP Technologies’ patented engineered Activ-Polymer™ technology can be an effective and efficient headspace management solution, addressing a wide range of desiccant and scavenging needs without the use of adhesives or purging, loose sachets or canisters, among other benefits.
CSP Technologies has developed Activ-Film™ and Activ-Blister™ materials to accomplish this efficient headspace management. CSP Technologies’ Activ-FilmTM technology can be used to control RH or oxygen levels, or to eliminate odors or reduce the levels of certain volatile organic compounds (VOCs) in a package. Solutions can be designed to solve a specific problem or address multiple issues simultaneously.
Similarly, CSP Technologies’ Activ-Blister™ solutions help control the internal atmosphere of existing individual blister cavities, allowing for improved product performance and enhanced shelf life. Offering moisture, oxygen and combination absorption, the technology can be applied without the use of adhesives and without changes to the existing footprint of a packaging line. Activ-Blister™ solutions can be incorporated into a wide range of blister packaging formats, including push-through blisters, peel/push blisters, cold-form foils and high barrier films containing Aclar®* laminates.
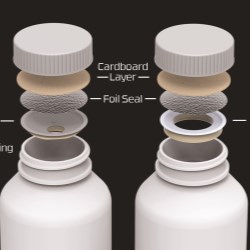
In addition, the company’s Activ-Seal™ closures are designed with desiccant technology press-fit into an induction sealed, tamper-evident screw cap. The closure’s orifice-reducing element with moisture and gas scavenging rests inside the bottleneck, and is permanently affixed to the top rim of the bottle via patent-pending effortless insertion technology during the induction sealing process. This closure uses commercially available, tamper-evident induction seals which, once removed, enable metered dosing. Once the cap is induction sealed to the rim of the bottle, the desiccant technology becomes part of the bottle and cannot be removed.
CSP Technologies, Inc. has decades of experience in providing custom polymeric and packaging solutions for companies around the world.




.jpg)
.jpg)





.jpg)
.jpg)












.jpg)



.png)