Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.

François Bidet, Director of Business Development EMEA for CSP Technologies, Inc. – a leader in packaging solutions that ensure product protection, enhance brand recognition and improve consumer experiences – will headline a session concerning pharmaceutical efficacy and compliance at the Smart Packaging 2017 conference, in Cologne, Germany.
Titled "Enhancing Shelf Life and Simplifying Packaging for Transdermal & Oral Solid Dose Drugs with Engineered Polymers for Head-Space Management," Mr. Bidet's presentation will showcase CSP Technologies' patented approach to tackling headspace challenges, including moisture and oxygen ingress, whose negative impacts can be detrimental to a pharmaceutical product's efficacy.
Despite being two very different drug delivery platforms, oral solid doses and transdermal patches can face similar packaging headspace challenges that impact shelf life; besides moisture and oxygen ingress, exposure to certain conditions can lead to the formation of volatile organic compounds (VOCs) and other gases that, in turn, may interact with drug products and capsules, adversely impacting their stability.
When utilized within pouch, blister and other small spaces, CSP Technologies can be an effective and efficient headspace management solution, addressing a wide range of desiccant and scavenging needs without the use of adhesives or purging, loose sachets or canisters, among other benefits.
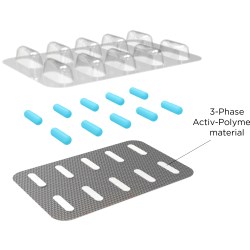
CSP Technologies has developed Activ-FilmTM and Activ-BlisterTM materials to accomplish this efficient headspace management. CSP Technologies' Activ-Film technology can be used to control residual humidity or oxygen levels, or to eliminate odors or reduce the levels of certain volatile organic compounds (VOCs) in a package. Solutions can be designed to solve a specific problem or address multiple issues simultaneously.
Similarly, CSP Technologies' Activ-Blister solutions help control the internal atmosphere of existing individual blister cavities, allowing for improved product performance and enhanced shelf life. Offering moisture, oxygen and combination absorption, the technology can be applied without the use of adhesives and without changes to the existing footprint of a packaging line. Activ-Blister solutions can be incorporated into a wide range of blister packaging formats, including push-through blisters, peel/push blisters, cold-form foils and ultra-high barrier films.
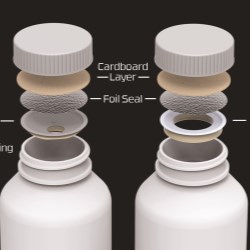
Another solution made possible by CSP Technologies' Activ-PolymerTM technology is the company's Activ-VialTM portfolio, a series of containers consisting of one-piece, flip-top desiccated vials and bottles across a range of sizes. Offering leak-proof sealing via an attached lid that cannot be lost or misplaced, Activ-Vial comprises an engineered desiccant sleeve whose moisture control literally surrounds the products for enhanced product stability. Container sizes range from 6-166 milliliters.
CSP Technologies, Inc. has decades of experience in providing custom polymeric and packaging solutions for companies around the world.




.jpg)
.jpg)





.jpg)
.jpg)












.jpg)




.png)