If this is your company, CONTACT US to activate Packbase™ software to build your portal.


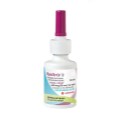
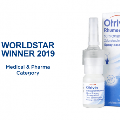
Aptar Pharma, part of AptarGroup, Inc. (NYSE: ATR), a global leader in drug delivery systems, services and active material science solutions, today announces the launch of APF Futurity, its first metal-free, multidose nasal spray pump developed to deliver nasal saline and other comparable over-the-counter (OTC) formulations.
Specifically designed for recyclability, APF Futurity is Aptar Pharma’s first highly recyclable nasal spray pump, having achieved a Class AA certification from cyclos-HTP for recycling streams in Europe[1]. APF Futurity is made from polyolefin materials with no metal parts or recycling disruptors, thereby minimizing separation efforts in recycling streams and supporting a higher quality of recyclates.
Gael Touya, President, Aptar Pharma, commented, “As a leading provider of nasal spray pumps globally, the launch of APF Futurity is a significant step forward in our sustainability journey, underlining Aptar’s overall commitment to a more circular economy and meeting the global need for sustainable packaging solutions that are easy to recycle.”
Part of Aptar Pharma’s Futurity sustainable solutions platform, APF Futurity meets a growing consumer need for increased access to recyclable products. In a study conducted by Aptar Pharma in 2022, 77% of respondents indicated that it was ‘important’ or ‘very important’ that the products they buy can be recycled[2]. When used in combination with a high-density polyethylene (HDPE) or polypropylene (PP) container bottle, a product with the APF Futurity pump can be conveniently recycled as one piece when empty after use, with no need to separate or detach any parts or materials before disposal, a key user benefit.
Innovative design based on proven technology
APF Futurity is based on Aptar Pharma’s proven Advanced Preservative Free (APF) technology platform, and has been initially developed to deliver multidose nasal saline and comparable OTC formulations. The innovative metal-free pump, for which patent protection has been filed, delivers precise and consistent dosing with soft actuation, with no trade-off on handling and performance.
“APF Futurity is a game-changing innovation for the nasal spray pump industry and I am proud of our design and engineering team for meeting the challenge of designing this metal-free nasal pump,” stated Stefan Ritsche, President Global Market Development, Consumer Health Care, Aptar Pharma. “This is an important step in the transformation of our nasal spray portfolio to more sustainable solutions, and we look forward to bringing additional innovations of this kind to meet the need in the pharmaceutical industry.”
Aptar’s commitment to sustainability
Driven by our commitment to sustainable practices and products, with a focus on delivering transformative solutions that improve our world, Aptar continues to shape the drug delivery and consumer product dispensing markets.
“Aptar continues to receive recognition for our sustainable product innovations, including the Institute of Packaging Professionals (IOPP) and WorldStar Global Packaging awards, and we were recently ranked #15 on Newsweek’s ‘America’s Most Responsible Companies‘ list,” added Christophe Marie, Product Sustainability Director, Aptar. “Aptar Pharma’s launch of APF Futurity is yet another sustainable product innovation that reinforces our pledge to care for our planet and reduce our environmental impact.”


.jpg)


.jpg)
.png)
.jpg)


 copy.jpg)


.jpg)
.jpg)























.jpg)


