If this is your company, CONTACT US to activate Packbase™ software to build your portal.


Until a few years ago, experts of the sector considered the days of dry powder inhalers (DPIs) – in the “single dose per capsule” format – to be numbered.
In their desire to find increasingly innovatory solutions, as well as to show themselves more technologically sophisticated than their competitors, many pharmaceutical companies had placed their bets on multi-dose inhalers with more advanced engineering and, on the face of it, simpler for the user.
In reality, many of these inhalers proved to be too complex technically and, in certain cases, unreliable, insufficiently user-friendly or too bulky.
The well-known failures of various highly sophisticated devices, which were expected to invade the market like blockbusters, brought about a return to favour of simpler, safer and more effective devices such as the single-dose per capsule inhaler.
Although these devices require a new capsule to be loaded before each inhalation, they nevertheless have a number of unquestionable advantages: the medicine is pre-dosed and the dose can be checked on the filling-lines; the patient can easily see, after each inhalation, whether the medicine has been taken; blister packaging ensures high stability of the medicine.
The capsule filling systems are furthermore standardized and reliable, thereby calling for relatively low investment by the pharmaceutical companies and allowing easier transition from the clinical, pre-industrial to the industrial phase.
As is well-known, Plastiape can boast today a thirty-year tradition in the development and industrialization of Inhalers for Powders. The producer of Aerolizer (Novartis Pharma), Pulvinal (Chiesi Farmaceutici) and Turbospin (PH&T), it also has several families of inhalers of its own design.
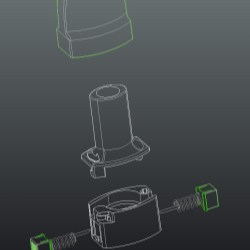
The latest generation single-dose per capsule inhaler is the Monodose RS01.
Compared with the more sophisticated multi-dose and other single-dose inhalers on the market, the RS01 is notable for its compact size, reduced number of parts and for the simplicity and effectiveness of its perforation system, suited to both gelatine and HMPC capsules.
The RS01 is created with thermoplastic resins specifically intended for the pharmaceutical sector and which have passed the physical-chemical tests laid down by international pharmacopoeias. With the collaboration of laboratories operating in GLP conditions, Plastiape has also performed extractible tests on its families of single-dose inhalers and biocompatibility tests on the finished product in compliance with ISO10993 regulations.
In its standard version, the RS01 is a low-resistance device reaching a pressure drop of 4 kPa at c.100 L/min and is therefore suitable for use on a wide range of patients. A high-resistance (4 kPa at 65 L/min) version also exists.
The inhaler is entirely developed by Plastiape and a number of international patents have been taken out for it (Europe, USA, Canada, Australia, Japan). Plastiape has also filed a Type III Drug Master File with the US Food and Drug Administration as a support for customers intending to register and trade their medicine on the American market.
The highly compact size, the lesser weight compared with competing appliances and the complete lack of propellants make the device particularly environmental friendly and therefore suitable for pharmaceutical companies that, like Plastiape, put respect for the environment at the top of their development strategy.
Plastiape has already developed high capacity moulds and production lines for the RS01 and can therefore provide its clients – even in the case of samples for clinical use – with devices produced in the same conditions as will be applied during industrial production.
The highly automated assembly lines are equipped with control stations designed to check all functional aspects of every single inhaler produced.
The device is assembled in a class IS0 7 white chamber (10,000 FED STD) subject to periodical checks for microbiological and particle contamination.
All the above-stated facts make the RS01 an ideal platform for a wide range of inhalant medicines: on the one hand, it may be considered a simple and cheap solution for generic medicines, even those produced in small volumes, while on the other hand it offers a reliable, ready-to-use solution for highly innovatory formulations, where it is necessary to limit the number of variables at stake, reducing the risks and costs of developing a device and thereby leaving researchers free to concentrate their efforts and investments on the formulations themselves.
















.jpg)


.jpg)











.jpg)










.jpg)





