
Bona, a high-tech enterprise located in Shenzhen, China was founded with the goal of providing excellent product solutions and customized drug delivery systems. With nearly 20 years of experience in developing, manufacturing, and marketing a variety of pharmaceutical dispensing systems worldwide, Bona is swiftly becoming one of the Asian region's leaders in the global dispensing system industry.
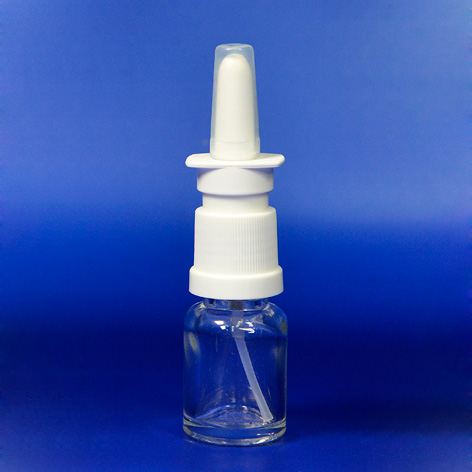
The company has launched a new patent product, a child resistant nasal spray bottle which has been designed specifically for nasal sprays which include imidazolines, according to the new regulation voted in by the US Consumer Product Safety Commission (CPSC).
The spray bottle has been tested by a CPSC recognized facility for the testing of child resistant packaging and administers the package evaluation under strict adherence to the Code of Federal Regulations (CFR) Title 16, Part 1700.20. In addition, the raw materials of the spray bottle are FDA compliant.
The child resistant nasal spray has a screw on neck facility and is designed to be paired with a ratchet neck bottle of 15ml or 30ml size. The nasal pump benefits accurate dosage from 50mcl to 170mcl per stroke and its screw on neck facilitates incorporation into existing filling lines easily.
Bona can also offer child proof eye dropper customization according to CFR 16 1700.20.
Bona Medicinal Packaging is a drug delivery system innovator and manufacturer with GMP Standard Class 100,000 and 10,000 clean room, and is both ISO9001:2008 and ISO14001 certified.