If this is your company, CONTACT US to activate Packbase™ software to build your portal.


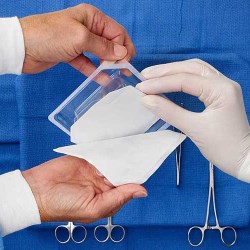
DuPont Protection Solutions announced the introduction of DuPont™ Tyvek® 40L medical packaging, a new class of Tyvek® for medical packaging applications that provides a cost-effective option for protecting lightweight, low-risk devices. This economical alternative to high-end medical papers was unveiled today at MEDTEC 2017 in Shanghai, China, where DuPont continues its year-long celebration of the 50th anniversary of Tyvek®. Commercial sales of Tyvek® 40L will begin immediately in China and there are plans for global availability in late 2018.
"The addition of Tyvek® 40L to our family of Tyvek® styles for medical and pharmaceutical packaging is the latest example of our long-standing and ongoing commitment to making a world of greater good possible," said Margaret Pyers, global business leader for DuPont™ Tyvek® Medical and Pharmaceutical Packaging. "Tyvek® 40L fills a previously unmet need for a better material to package lightweight, low-risk Class I and IIa devices, especially in emerging Asian markets," explained Pyers.
"We are proud to continue our long tradition of meeting marketplace needs with innovative products that provide trusted protection so people can accomplish bigger things."
DuPont Expands Family of Tyvek Styles for Medical Packaging to Meet Marketplace Needs
Tyvek® 40L features many of the same benefits that have made Tyvek® a standard of excellence for medical packaging since 1972, including: maintaining sterility until point of use – even under rigorous conditions; low particulate generation; clean peel; puncture and tear resistance during handling and transportation – even in high humidity; and compatibility with most major sterilization methods. This lighter weight Tyvek®, which offers excellent performance with ethylene oxide (EO) and gamma radiation sterilization, can be readily distinguished by its embossed, linen-like appearance.
When compared to reinforced, heavy basis weight papers, Tyvek® 40L shows better strength and far superior breathability. Tyvek® 40L also peels cleanly when opening the package, even if the package is uncoated and sealed to a peelable film. With its very low basis weight, Tyvek® 40L provides excellent value and packaging source reduction opportunities to support sustainability efforts.
In addition to offering a range of outstanding microbial barrier products, which now includes Tyvek® 1073B, Tyvek® 1059B, Tyvek® 2FS™ and new Tyvek® 40L, the DuPont Medical and Pharmaceutical Packaging Team is dedicated to providing technical support to medical device manufacturers (MDMs) around the world, sharing information and expertise on topics ranging from industry standards and regulatory compliance to technical issues and quality.
Members of the team also play key roles in industry associations and standards-setting organizations, such as Association for the Advancement of Medical Instrumentation (AAMI), European Committee for Standardization (CEN), International Organization for Standardization (ISO) and Standardization Administration of the People’s Republic of China (SAC), to name a few.
For more than 45 years, Tyvek® has helped to protect the health of millions of patients worldwide and to reduce costs in the health care setting by maintaining sterility of medical devices and supplies to the point of use. Recognized as a standard of excellence in health care packaging, this advanced material is used in virtually every form of sterile medical packaging, as well as a wide variety of pharmaceutical packaging applications.
For Greater Good™ is the promise of the DuPont™ Tyvek® brand. Tyvek® can provide the trusted protective barrier people need to worry less so they can focus on accomplishing bigger things – making the greater good possible.