Public
Lameplast 3D Catalog
Lameplast Catalog
Lameplast Documents
Lameplast Gallery
Lameplast Locations
Lameplast News
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


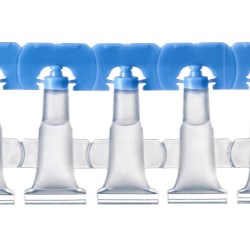
The Lameplast system features pre-made vials that provide a higher level of precision, functionality and quality control compared to blow/fill/seal systems. The vials offer a more attractive physical appearance (no burrs), uniform orifice/drug delivery, flexible closure design and printing capability, ability to mold with barrier materials vs. only one material, more uniform wall thickness, and reclosable features. These are all attributes that are not possible when the unit dose vial is blown, filled and sealed in one stage.
Lameplast will also be discussing its semi-automatic and fully automatic Pentafill™ fill/seal machines. Applications include pharmaceutical, diagnostic, medical device, veterinary and cosmetic applications, with an emphasis on vaccines and ophthalmic medicine.
The business unit’s quality management system is ISO 15378 and ISO 9001 certified. Lameplast is also a CE-mark holder for Class I medical devices, which indicates compliance with applicable European Union (EU) regulations and enables the commercialization of products in the 32 EU countries. Production is carried out in Class ISO 7 (Class 10,000) and ISO 8 (Class 100,000) controlled contamination environments according to ISO 14644-1.
Lameplast, a Tekni-Plex business, will be showing its line of plastic single and multidose containers, and filling equipment, for pharmaceutical/healthcare applications at Pharmapack, February 5-6, Paris Expo, Porte de Versailles, Paris, Booth E16.