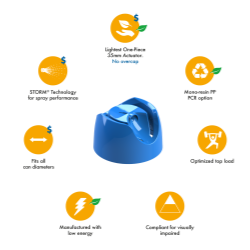
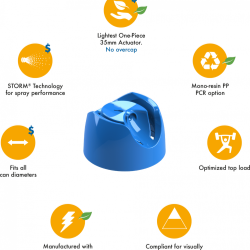
We are pleased to announce that Precision Global has been awarded ISO 13485 certification by the American Systems Registrar in recognition of our ability to meet stringent regulatory standards and requirements for the manufacture of aerosol valves for use in the medical industry.
The certification was earned through an audit of Precision’s clean room facility located in Greenville, SC. This clean room specializes in manufacturing medical dispensing solutions for pharmaceutical customers. It was previously certified in 2014 under US Federal Standard 209E Class 100,000, requiring the facility to adhere to a strict set of safety and hygiene standards.
This accreditation strengthens our ability to offer innovative, medical-grade aerosol valve solutions to the growing medical device and pharmaceutical industries. It also empowers Precision to enter new medical valve markets, including the use of aerosols in injectable and insertable forms. We are excited about what this means for us and our valued partners and customers.