2024년 최신 슬롯사이트 순위 TOP10 소개
Generate Custom Oligo Synthesis: A New Era in Transportation Attributes
Telegram下载:通信新时代的开启
APK Download: Unlocking the Potential of Mobile Apps Beyond the App Store
Max Concrete Services: Crafting Durable Foundations for the Future of Phoenix
Peace Haven Jewellery: A Sparkle of Serenity in Every Piece
The Art and Science of Book Cover Design: Capturing the Reader’s Attention
The Future of Sustainability: PLA Fiber and its Role in the Non-Woven Fabric Industry
The Importance of an Emergency Electrician: How Max Electric Services Keeps Houston Safe
Top Influencer in Crypto: Shaping the Digital Currency Landscape
Toronto Digital Marketing: How WebDeveloperInToronto.com Powers Business Success Online
Unlocking Success: Starting Your Own CNY Cookie Business in Malaysia
Unveiling the Best Cash for Cars Brisbane: Your Ultimate Guide to Hassle-Free Vehicle Selling
14 x 14 ft Insulated Garage Doors
200 Hour Yoga TTC in Rishikesh: Unlock Your Full Potential with HimalayanYogAshram
96 Inch Sliding Patio Door: The Perfect Addition to Your Home by WarrenWindows
A Comprehensive Guide to Disposable Pods: Convenience and Simplicity
A Comprehensive Guide to Pod Systems: What You Need to Know
Abogado Accidente de Tráfico: ¿Por qué es Fundamental Contar con el Mejor Abogado en tu Reclamo?
Abogado Barcelona Penalista: Tu Defensa en Casos Criminales
Abogado de lo Penal: Su Aliado en Momentos Difíciles
Abogado Penalista en Barcelona: La Mejor Defensa con Barna Legal Defensa Penal Barcelona
Abogado Penalista: Su Defensa Legal en Barcelona
Abogado Segunda Oportunidad Barcelona: La Solución Legal para Cancelar tus Deudas
Accelerate Your Website's Growth with a Free Indexing Service
Acheter de la morphine : Ce que vous devez savoir avant de faire votre achat en ligne
Acheter des Abonnés TikTok : Un Guide Complet pour Booster Votre Présence en Ligne
Adatlistazo.hu – A Mindennapi Élet Minden Területére Kiterjedő Online Magazin
Adba Machinery LLC: Your Trusted Partner for Top-Quality Equipment Rentals
Affordable Movers Ashland MA: Making Relocation Stress-Free
African Food Near Me: Exploring Flavors, Culture, and Connection
African Food Near Me: Exploring Flavors, Culture, and Connection
Agencia de Redes Sociales: La Clave para Impulsar tu Marca al Éxito Digital
Alt du Trenger å Vite om Boksehansker: En Komplett Guide fra HighWhey
Anatomical Model Manufacturer: Revolutionizing Medical Education
Apartment for Sale in Markham: The Ultimate Guide to Finding Your Dream Home with Homsy
APK Download: Unlocking the Potential of Android Apps with apkhihe
Atlanta Slip and Fall Accident Lawyer: Your Guide to Legal Rights and Protection
Attic Insulation Austin: Why It's Essential for Your Home's Comfort and Efficiency
B2B Company Insights: Unlocking the Power of Business Intelligence with Financh
Bật Mí Những Kinh Nghiệm Chơi Rồng Hổ Bất Hủ Cho Người Mới
Bật Mí Những Kinh Nghiệm Chơi Sicbo Hiệu Quả Nhất Hiện Na
Bath Remodeling Wellington: Transform Your Space with RenovisionKB
Beli Akun Facebook MCY Agency: Menjaga Keamanan dan Memaksimalkan Potensi Online Anda
Best Commercial Cleaning Services in Pickering: Why Elite Cleaning Services Stands Out
Best Mod Menu for GTA V in 2024: Elevate Your Gaming Experience with Stand Mod Menu
Best Pet Shop in Dubai: FurryBabiesDubai – The Ultimate Destination for Your Pet’s Needs
Best Quotex Review: An In-Depth Look at a Rising Trading Platform
Best Tech Accessories: Enhancing Your Digital Experience with the Latest Gadgets
Betpawa TZ: A Safe and Responsible Gambling Experience with Sokabet
BetRanking.pl – Twoje Niezastąpione Źródło Wiedzy o Zakładach Sportowych i Kasynach Online
Book Cover Design: Captivating the Reader at First Glance
Boost Your Online Presence Using OnLinker: The Smart Way to Purchase Website Traffic
Boost Your Streaming Success with ViewerBoss: The Ultimate Kick Viewer Bot
Boost Your Streaming Success: A Deep Dive into Kick Bots Viewers with ViewerBoss
Boosting Trading Volume with the Solana Volume Booster Bot: A Game Changer for Crypto Traders
Buy Bong Online: Your Ultimate Guide to Finding the Perfect Piece at 420cannabisway
Buy Cigarettes Online: A Seamless Shopping Experience with Tobaccoash
Buy HEETS Online: Your Guide to Enjoying a Premium IQOS Experience
Buy THC Gummies Australia: Elevate Your Experience with Weed Delivery Australia
Buy Valorant Accounts: Elevate Your Gaming Experience with CSGOSMURFNATION
Buy Weed Online Australia: Your Ultimate Guide to W.D.A.
Calmly Navigating the World of Tracker Recruitment Software
Captivating Customers with Metal Display Showcases: The Ultimate Solution for Retail Success
Car Hire with Driver Marrakech: The Ultimate Way to Explore the Red City
Carpet Cleaning Near Me: Your Go-To Solution for Spotless Spaces
Ceramic Coating Toronto: The Ultimate Protection for Your Vehicle’s Paint
Cheap Cigarettes Online: Your Ultimate Guide to Affordable Smoking
Cheap Cigarettes: An Evolving Market and Alternatives
Chinawindoors Windows and Doors: A Look into Modern Solutions for Your Home
Choosing the Right LED Module Suppliers for Your Projects
Christmas Lights in Orlando: A Magical Holiday Experience
Cigsheated: Where Innovation Meets Pleasure in the World of Heat-Not-Burn Technology
Circuit Thaïlande du Sud : Un Voyage Inoubliable au Cœur des Paradis Cachés
Close Coupled Back To Wall Toilet: The Ultimate Bathroom Upgrade
Closed Loop Motor: Enhancing Efficiency and Precision in Nevada
Comparative Analysis of Electronic Paper Display and Competitive Pricing Strategies
Comprehensive Tree Services in Druid Hills: Enhancing the Beauty and Safety of Your Property
Constantly Advancing: The Legal and Regulatory Landscape of Bilateral Hearing Loss Implants
Contemporary Dining: The Perfect Blend of Style, Comfort, and Functionality
Cooking Team Building: A Recipe for Engagement and Collaboration
Crafting Success: How a Cover Letter Builder Can Transform Your Job Application
Cryptojacking Explained: How To Protect Your Business From Hidden Mining Attacks
Custom Cigar Bags: Elevating the Cigar Experience
Custom Sculptures: The Art of Personalized Décor
Das Ultimative Solitär Erlebnis: Warum unser Solitär# Browser Spiel die beste Wahl ist
Decentralized Masters Review: Is This Crypto Opportunity Legit?
Deneme Bonusu Veren Siteler: Unlocking the Benefits of Trial Bonuses
Diamond Estate Services: California’s Top-Rated Estate Sale Company
Diesel Scissor Lift Elevates Maintenance Standards
Dinar Detectives: Unveiling the Best Deals for Currency Exchange with Dinarit
Disability Insurance Lawyers: Fighting for Your Rights with Eric Buchanan and Associates
Discover New Realms of Gaming: A Deep Dive into the Latest on PlayStation, Xbox, and Nintendo Games
Discover the Art of Beauty at M Beautique Salon: Where Your Style Meets Perfection
Discover the Best Airsoft Stores: Your Guide to the Ultimate Airsoft Experience
Discover the Best Bathroom Vanities at Pure-Design: Quality, Durability, and Style
Discover the Joy of Crochet: Free Crochet Patterns in PDF
Discover the Magic of Paso Robles Wine Tours
Discover the Magic of Paso Robles Wine Tours with Elegant
Discover the Magic of Playtime: Shop Irresistible Toys for Kids of All Ages at Whitcoulls!"
Discover the Perfect Townhomes in Riverview: A New Era of Comfort and Convenience
Discover the Top Online Deals: Prime Day Bargains and Electric Bike Discounts Await
Discover the Ultimate Workshop Gear: Shop Essential Tools and Equipment at TopmaQ
Discover the World of PIFF CARTS: The Ultimate Vaping Experience
Discover the World with FoxLTR: Your Travel Companion for Unforgettable Adventures
Discover WDMA: Revolutionizing the Windows and Doors Export Industry
Discovering "Deneme Bonusu Veren Siteler": Your Gateway to Free Betting Bonuses
Discovering Gas Kids 4 Wheelers for Sale: The Ultimate Guide for Young Adventurers
Discovering Marlboro HEETS: A Modern Solution for Smokers
Discovering the Best Perth Curtain and Blinds Shop for Your Home
Discovering the Best Scooters for Sale Near You: A Guide to Q9 Power Sports
Discovering the Heets Shop: Your Gateway to a Revolutionary Tobacco Experience
Discovering the World: A Guide to Booking Your Next Flight, Hotel, and Trip
Discovering Tobaccoash: The Premier Online Tobacco Shop for Avid Smokers
Discovering WarrenDW: Elevating Home Comfort with Innovative Window Solutions
Discovering WarrenWD: A Leader in Windows and Doors Manufacturing in China
Disneyland Tickets Günstig: How to Find Affordable Magic for Your Family
DJ Taydeville: The Ultimate Collaboration with Crime Mob's Lil Jay
DoldAdress: Säkerhet och Integritet i En Digital Värld
Dom Pogrzebowy Warszawa: Jak Wybrać Najlepszą Usługę W Warszawie?
Doorwin Windows: Revolutionizing Modern Home Design
EBM Avenue: Paving the Way for a Sustainable, Inclusive, and Eco-Friendly Future
Effortless Moves with TDN Man and Van: Your Ultimate Guide to Removals in Peterborough
El Paso Personal Injury Lawyer: Advocating for Justice and Results
Elevate Your Home: The Essential Guide to Windows and Doors
Elevate Your Space with Warren Floor-to-Ceiling Windows
Elevate Your Spaces with Chrimson: The Authority in Windows and Doors
Embark on an Unforgettable Journey with DesertTrips: The Ultimate 3-Day Sahara Desert Tour
Embrace the Future of Home Design with Bi-Folding Doors
Embracing Sustainability: A Green Orbit Approach to a Greener Future
Embracing the Elegance of Narrow Long Skinny Windows That Open
Empowering Your Career Journey: How a Seamless CV Builder Can Make a Difference
Enslaving men ... seen as a ... hobby by the well known romanian author Adrian Dumitru
ESWDA: Your Go-To Authority for Quality Windows and Doors
Everything You Need to Know About 6x6 Windows: Size, Benefits, and Considerations
Everything You Need to Know About 7 Ft Garage Doors
Executive Hiring for Consumer Goods: Building Leadership in a Dynamic Market
Experience Luxury Living with Sentosa Beachwalk: Your Ultimate Beach Walk Rentals Destination
Experience the Best of Paso Robles Wine Tours: A Journey through California’s Wine Country
Experience the Magic of a Private Angkor Wat Sunrise Tour
Experience the Royal Touch with ITSU’s Massage Chairs
Expert Stump Removal in Devonport: Why Prime Auckland Tree Service is Your Go-To Solution
Expert Tree Service in Acworth, GA: Your Trusted Partner for Tree Care and Removal
Exploring & Looking into the Benefits of Overmolded Cable in Supply Chain Management
Exploring APK Games: A Gateway to Endless Fun and Convenience
Exploring Brooklyn Interior Doors: Elevate Your Home's Style with Doors & More
Exploring Hurghada's Real Estate: A Treasure Trove of Luxurious Living
Exploring MLS Richmond Hill: A New Era in Real Estate with Homsy
Exploring the Advancements of Robot Mobile Platforms
Exploring the Allure of Korean Plastic Surgery: Beauty Redefined at MINE Plastic Surgery
Exploring the Future of AI Video: Revolutionizing Content Creation
Exploring the Future of AI with HiheAI.com: Your Gateway to the Best AI Tools
Exploring the Role of Compact Devices in Modern Lifestyles
Exploring the Transformative World of Czech Tantra Massage
Exploring the Wonders of Desert Trips Morocco
Exploring the Wonders of Polarized Beam Splitter
Exploring the World of Premium Cannabis with 420cannabisway
Exploring the World of Premium Tobacco with CigarettesRoad
Exploring the Z-Library Project: Revolutionizing the Future of eBooks
Exploring Tobacconear: Pioneering a Smoke-Free Future with IQOS
Exploring Weird Topics: Unveiling the Oddities of Our World and Beyond
Export Trade: Unlocking New Opportunities for African Foodstuff Exports with Humblesage Food
Facelift: Transformasi Kecantikan dan Kepercayaan Diri di Klinik Bedah Plastik MINE
Fake AI Influencers: The Future of Marketing?
Finansnet: Din Komplett Ressurs for Finans og Investeringer
Fincas Bodas Zaragoza: La Elección Perfecta para un Día Inolvidable
Finding the Best Physio Near Me: Your Guide to Physiotherapy in Abu Dhabi
Finding the Best Plumber in Sherman Oaks: Your Guide to Top-Notch Plumbing Services
Finding the Perfect Curtains for Your Home: Your Ultimate Guide to Hay Interiors Curtain Shop
Finding the Perfect Realtor Near Me: A Guide to Success
Finding Your Dream Home with MLS Toronto: A Guide to Efficient Home Search
Flashlight Stun Batons: The Ultimate Self-Defense Tool
Flower Delivery Gatineau: Bringing Joy to Your Doorstep
Foot and Ankle Health: Understanding, Preventing, and Treating Common Conditions
From Subtle to Statement: How Your Ring Style Can Evolve Over the Years
Furniture Removal in Ahwatukee: A Comprehensive Guide to a Smooth and Stress-Free Process
Garage Door Repair in Burnaby: Ensuring Safety and Efficiency with Comfort Doors
Gear Motor Manufacturers: A Comprehensive Guide to Value
Get Your NIE in Barcelona: A Comprehensive Guide
Grout Cleaning Gold Coast: Bringing Back the Shine to Your Tiles
Hệ Thống POS Cho Tiệm Nails: Giải Pháp Toàn Diện Đến Từ Pionails
Heater Repair in Austin: Ensuring Comfort and Efficiency with Grande Air Solutions
Helium 10 Free Trial: Everything You Need to Get Started Today
Hemligadress: Skydda Din Personliga Information Online med DoldAdress
Holowanie w Rudzie Śląskiej: Wybór Idealnej Firmy do Pomocy na Drodze
Houston Vending Machine Service: The Key to Enhanced Workplace Convenience
How automated data entry solutions benefit the government sector?
How Buying TikTok Followers Can Boost Your Social Media Game: A Smart Strategy for 2024
How Much is David Barton Gym Per Month? A Look Inside One of New York’s Most Exclusive Gyms
How the Solana Volume Bot is Revolutionizing Trading on DEXes
How to Buy Property: The Ultimate Guide to Making Your Dream Home a Reality
How to Convert Bank Statement PDF to Excel: A Comprehensive Guide
How to Convert PDF to CSV Bank Statements: A Comprehensive Guide by StatementSheet
How to Download Instagram Videos for Free Using InstaFreeVideoDownload
How to Earn Affiliate Income with AI: A Guide to Making $100/Day with ClickBank and ChromeWebStore
How to Get Gems in Brawl Stars: A Guide to Unlocking In-Game Currency
How to Grow X (Twitter) Followers: A Complete Guide for Content Creators
How to Harness Robotic Control Systems in Connecticut for Your Next Big Project
How to Have the Expertise to Perform Exceptional Client Service
How to Manufacture Zirconium Oxide Ceramics?
How to Memorize the Quran Online: A Comprehensive Guide to Achieving Your Goal
How to Overcome Approach Anxiety: Unlock Your Confidence and Master the Art of Attraction
How to Transfer Zoom Recordings to Another Account
How Working Past 65 Impacts Your Medicare Choices
Hurghada Real Estate: A Lucrative Investment Opportunity on the Red Sea
Hydra Pen: The Future of Skin Rejuvenation
Idyllic Freezer Inventory Management: A Comprehensive Overview in the Taiwanese Market
Impressive Innovations from Major Battery Manufacturers: Powering the Future with a Smile!
IMR 4320 Powder: The Reloading Powder of Choice for Precision and Versatility
Index Checker Online: Unlocking Your Website’s Full Potential with SpeedyIndex
Innovations in Fertilizer Automatic Batching Systems and ODM
Innovative Phone Case Heat Press Machine: Enhancing User Experience
Instalador Electricista: ¿Por Qué Elegir a Electricistas Express Bilbao para tus Emergencias Eléctricas?
Investir en Bourse : Votre Guide pour Devenir Plus Riche
Iraqi Dinar for Sale: A Guide to Understanding Its Market and How Dinarit Can Help
Junk Removal: Simplify Your Life with Don't Move A Muscle
JY娛樂城:全球華人線上娛樂的頂尖平台
JY娛樂城:探索全方位的娛樂體驗
Kaboom77 Login: Your Gateway to Australia’s Premier Pokies Destination
Katy Wrongful Death Lawyer: Advocating for Justice in Your Time of Need
Keigo Miura Exposed: The Fake Pilot Deception Unveiled Amid a Web of Drug Abuse and Fraud
Làm thế nào để đánh lô đề trúng lớn cho anh em lô thủ
Laneige: Revolutionizing Skincare with Hydration Innovation
Law Firm Marketing: Bridging the Gap Between Advertising and Real Solutions
Law Firm Marketing: Bridging the Gap Between Advertising and Solutions
Law Firm SEO: How Milemark Bridges the Gap Between Advertising and Real Solutions
LED Module Suppliers: The Bright Future of Sustainable Lighting Solutions
LG Fridge Water Filter NZ: Why It’s Essential for Your Home and How to Choose the Right One
Lingam Massage in London: A Journey of Sensual Relaxation
Link Slot Gacor: Kunci Menuju Kemenangan Besar di Dunia Slot Online
Liposuction: A Comprehensive Guide to Body Contouring at MINE Plastic Surgery
Lobe Pumps: The Unseen Workhorse Behind Industrial Success
Logopeda Infantil en Barcelona: Un Enfoque Integral para el Desarrollo del Lenguaje Infantil
Lost Love Solution: Rekindling the Flames of a Faded Relationship
Marlboro Cigarettes: The Perfect Blend of Bold Flavor and Tradition
Marshall Price in Sri Lanka: Your Guide to Premium Sound at iRiver.lk
Mastering Email Marketing: Expert Tips for Success
Mastering ICAO Level 5: Unlocking Global Aviation Careers with Level6Aviation
Mastering the MySQL LIMIT Clause: How to Optimize Data Queries and Boost Efficiency
Mastering the Singapore PR Renewal Process: A Comprehensive Guide
Maximizing Land Management: Essential Equipment for Effective Soil Preparation with Ripping It Outdoors
Maximizing Your Plant Growth with 500-Gallon Grow Bags: The Ultimate Guide
Maximizing Your Sales and Marketing Potential: ITScope’s Guide to Transforming Your Strategy
Membentuk Jawline Sempurna: Solusi Modern dari Klinik Bedah Plastik MINE
Mengatasi Double Chin dengan Klinik Bedah Plastik MINE: Solusi Terbaik untuk Penampilan Lebih Tirus
Mengoptimalkan Kontur Wajah dengan Perawatan Jawline di Klinik Bedah Plastik MINE
Menyelami Dunia Mega888: Pengalaman Kasino Dalam Talian yang Tak Terlupakan
Menyelami Dunia Taruhan Online di Milan69
Mesin Scrubbing Grosir: Solusi Pembersihan Efisien dari Wuxi Younis Cleaning Equipment Manufacturing Co., Ltd
Milan69: Fasilitas Game Online Terbaik untuk Pengalaman Bermain Tanpa Henti
Milan69: Platform Taruhan Online Terbaik untuk Kemenangan Besar
MLS Listings Toronto: Your Gateway to Finding the Perfect Home
Mnemba Island Snorkeling Trip: An Unforgettable Adventure in Zanzibar
Modern Interior Design Ideas: Transform Your Space with Style
Modern Philosophy: Navigating the Complexities of Contemporary Thought
Mold Restoration: Bringing Your Property Back to Life with Restoration 1
Motorised Curtains: The Future of Home and Office Window Treatment
Movement: A Force for Change at Girls For A Change (GFAC)
Movers and Packers in Dubai: Your Ultimate Guide to a Stress-Free Move with Shahi Movers
MPO1881: Kemudahan Deposit Slot QRIS Tanpa Potongan dengan Archiphic
Mpo1881: Situs Slot Deposit Pulsa Terpercaya untuk Pengalaman Bermain Gacor
Navigating Employment Law in the UK: Understanding Key Changes and Their Impact
Negozio Orologi Torino: Il Lusso a Portata di Mano con FGWatches
Nepal Trekking Packages: Your Gateway to Adventure with Trip Like Local
Niti Endodontic Files: A Comprehensive Study
No Cure No Pay SEO: De Toekomst van Zoekmachineoptimalisatie
OLXTOTO: Platform Premium untuk Penggemar Permainan Togel di Indonesia
Oneiric Shard: A Game Analysis
One-Off Financial Advice: A Smart Choice for Perth Residents
Operasi Plastik Korea: Memahami Fenomena Kecantikan yang Mendunia
Oregon City Personal Injury Lawyer: Your Guide to Legal Support After an Accident
Panduan Lengkap Memilih Agen Taruhan SBOBET Terpercaya: Net88 Menyediakan Solusi Terbaik
Panorica - Hybrid & Electric Cars: Driving Towards a Sustainable Future
Pergola for Sale: Transform Your Outdoor Space with Pergomatic
Personal Wellness: Exploring New Horizons with Lemonly
Pet-Friendly Apartments in St. Johns, FL: Your Ultimate Guide
PG Zeed: ประสบการณ์การเล่นสล็อตออนไลน์ที่ไม่เหมือนใคร
Pharmaceutical Testing: Ensuring Safety, Efficacy, and Quality in the Healthcare Industry
PictureWindow: Revolutionizing the Windows and Doors Market
Plumber Brooklyn: Why AC Drain Cleaning LLC is the Best Choice for Your Plumbing Needs
Portland Premises Accident Lawyer: Advocating for Your Rights
Portland Premises Accident Lawyer: What You Need to Know
Pourquoi Choisir une Agence Web à Chartres pour votre Projet Numérique ?
Power Washing Company in West Chester: How PrettyManPainting Transforms Your Home
Prime Auckland Tree Service: Your Trusted Partner for Quality Tree Care
Privat Middag med Kok: Den Ultimative Gastronomiske Oplevelse i Dit Eget Hjem
PROROOFUSA: Your Premier Roof Installation Company in Palm Beach
Protect Your Investment: The Ultimate Guide to Paint Protection Film (Xpel)
Quality Control Solutions: Empowering Businesses to Thrive
Quay Nổ Hũ Trên rikvip - Chơi Game Nhận Thưởng Jackpot Hàng Tỷ Đồng
Queen Anne Gutter Cleaning: Protect Your Home from Water Damage
ReadyBuiltBusiness.com – Investments: A Comprehensive Guide to Smart Investing
Real Estate Marbella: A Dynamic Investment Destination on the Costa del Sol
Real Estate Photography: Capturing the Essence of Property
Rediscovering Yourself with Disposable Vape Pens: A Guide to Enjoying Life with Lost Mary
Refine: The Transformative Impact of Cloud Computing Expo on Transportation Attributes
Reparación de Neveras: Todo lo que Necesitas Saber
Reparación de Televisores: Soluciones a los Problemas Comunes de tu Pantalla
Request for Proposal Software: Revolutionizing the RFP Process with BidWizard
Residential Construction Services in Del Mar, CA: Crafting Dream Homes with Integrity and Excellence
Revolutionising Networking with Digital Business Cards: Why You Need ICV.Bio
Revolutionize Workforce Engagement with an Employee Communication App
Revolutionizing Precision: The Future of Grinding Machine Solutions
Revolutionizing the Background Check Process: The GCheck Background Check
Revolutionizing Warehouse Operations with AMR Fleet Management
Roller Insect Screen Doors: The Ultimate Solution for Pest-Free Living
Rolls-Royce Vintage for Weddings: A Regal Touch to Your Big Day
Samsung Screen Repair: Why iRepair Experts is Your Trusted Solution
Santa Rosa Wrongful Death Attorney: Your Ally in Seeking Justice
Sculpting the Perfect Jawline: Aesthetic Trends and Modern Solutions
Seeking Justice for Loved Ones: Your Guide to Hiring a Santa Rosa Wrongful Death Attorney
Shine Bright, Mom: Embrace Your True Self with Jescojes
Situs Togel Terbaru: Menyediakan Pengalaman Bermain Togel Terbaik dan Terpercaya
Skandia Kollektiv: More Than Just a Hair Salon in Salt Lake City – A Journey of Empowerment and Transformation
Slidell Moving Company is Ready to Make Your Move Easy
Slot Gacor: Menemukan Kunci Kemenangan di Dunia Slot Online
Slot QRIS: Metode Deposit Murah dan Cepat untuk Bermain Slot Online
Slot QRIS: Revolusi Deposit Mudah dan Cepat di Dunia Slot Online
Slot: Hiburan Seru dengan Peluang Gampang Menang Bersama JANJIGACOR
Slot88 Resmi: Pengalaman Slot Online yang Menggembirakan dan Aman di MAUSLOT88
Small Woodworking Shop Layout Plans: Crafting Your Ideal Workspace
Solana Volume Booster: Unlocking the Power of Blockchain Scalability
Sophrologue à Paris : Découvrez la méthode douce pour gérer le stress et l'anxiété
Spaceman Slot: Menyelami Keseruan Permainan Slot Jackpot Terbaik
Spontaneously Illuminating the Future: The Rise of Street Light Roads in East Africa
Starting an LLC – Essential Steps, Benefits, and Considerations
STD Test at Home: Empowering Your Sexual Health with Convenience and Privacy
Steel Toe Rain Boots: A Perfect Blend of Protection, Comfort, and Durability
Streaming Layarcinema89: Menyelami Dunia Hiburan dengan Akses Mudah dan Menyenangkan
Streamline Your Ride-Share Vehicle Inspection with Insve: The Future of Uber Online Inspections
Sugar Defender Official: Revolutionizing the Way We Manage Our Health
Sugar Land Psychological Associates: A Beacon of Hope in Texas
Sunset Walk in Kissimmee: A Serene Experience at The Retreat at Sunset Walk Orlando
Supporting Mental Health in Texas: Sugar Land Psychological Associates Leads the Way
Tanah Lot Tour: Exploring Bali’s Iconic Sea Temple with TripPlannerIndonesia
Tech Trends: Are Long Sleeve Work Tops the New Fashion Statement?
Telegram下载:探索隐藏功能与安装技巧
Telegram中文版:数字交流的未来
The 10 Best Tarot Card Books to Deepen Your Spiritual Journey
The 48 Inch Window: A Modern Solution for Your Home and Office
The Art and Importance of Book Cover Design: How CreativeParamita Makes It Stand Out
The Art and Science of Vodka Distillation: Unveiling the Secrets Behind Premium Spirits
The Art of Book Cover Design: Capturing Stories in Visuals
The Art of Buying Cigarettes Online: Elevate Your Experience with Cigsking
The Art of Designing a Contemporary Living Room: A Guide to Modern Comfort and Style
The Art of Printed Socks: A Trendy Accessory for Comfort and Style
The Benefits of Nylon and Spandex Material
The Best eBikes for Adults: A Comprehensive Ride Review
The Charlie Brown: A Platform for Provocative Debate and Critical Thinking
The Craft and Innovation Behind Leading Sweater Manufacturers: A Deep Dive into DGJIAYAN’s Expertise
The Definitive Guide to Cheap Cigarettes: Quality, Variety, and Satisfaction
The Disposable Pod System: A Game-Changer in the World of Vaping
The Elegance and Functionality of Brass Coat Hooks: A Timeless Addition to Your Home
The Elegance and Practicality of Perth Sheer Curtains: A Perfect Window Treatment for Every Home
The Essential Guide to Grafted Clips: A Game-Changer for Modern Agriculture
The Essential Guide to Hiring a Car Accident Lawyer: What You Need to Know
The Essential Guide to Hiring an Atlanta Divorce Lawyer
The Essential Guide to Junk Removal: Decluttering Your Life
The Essential Role of Insulation Cutting Machines in Modern HVAC Systems
The Ethereum ICO: A Game-Changer in Blockchain Development and How Reploy is Shaping the Future
The Evolution and Future of Modern Blogging: A Comprehensive Guide by Haporium
The Evolution of Smoking: IQOS HEETS and the Future of Tobacco Consumption
The Evolution of Windows and Doors: A Deep Dive into RenewalByWarren
The Expedition of Wholesale Curtain Poles
The Fascinating World of Mushroom Chocolate: A Delicious Trend with a Twist
The Future of Construction: How BIM Services in UAE Are Revolutionizing the Industry
The Future of Convenience: Innovative Gadgets That Transform Everyday Life
The Future of Custom Apparel: The Power of the Automatic Rotary Socks Printer
The Future of Document Management: How EasyFill is Revolutionizing Fillable Forms
The Future of Learning: How Technology is Shaping the Chess Ecosystem
The Importance of Book Cover Design: How It Influences Success
The Importance of Chimney Cleaning: A Guide to Safety and Efficiency
The Importance of Finding a Manhattan No-Fault Physical Therapist
The Importance of Non-Slip Floor Treatments for Safety and Durability
The Importance of Professional Investigative Services in a Complex World
The Importance of Professional Investigative Services in a Complex World
The Importance of Quality Soft Play Equipment for Indoor Playgrounds
The Importance of SQE Notes for Aspiring Solicitors: A Guide to Success
the kind wash: Professional Cleaning Services & Laundry Solutions for Shoes, Bags, and Baby Gear
The Korean Face Lift: A Modern Solution to Timeless Beauty
The Last of Us: A Timeless Masterpiece in Gaming
The Legacy of Marlboro Cigarettes: A Closer Look
The Legacy of Marlboro Cigarettes: Why Marlboro Red Remains a Timeless Choice
The Magic Behind Creative Agencies: Why Your Brand Needs One to Stand Out
The Mini Facelift: A Revolution in Age-Defying Beauty
The Perfect Single Bed for Every Sleepyhead: Discover Smiths City's Expert Advice on Styles, Sizes, and Comfort
The Polyester Fabric Jacket: A Smart Choice for Style, Comfort, and Durability
The Power of a Global Data Platform: How Financh is Revolutionizing Business Intelligence
The Power of B2B Marketplace Platforms: How GlobalTradeAxis Is Redefining International Trade
The Power of Book Cover Design: Why It Matters for Your Book's Success
The Power of Inner Growth: Unlocking Your Full Potential
The Power of Precision: Exploring Alliant Bullseye Gun Powder
The Power of Pressure Washing: Transforming Your Home and Business
The Power of Rejuran: Transforming Skin Health with Cutting-Edge Technology
The Power of Website Design: How a Professional Web Presence Drives Business Growth
The Power Socket: A Vital Component of Modern Life and What You Need to Know
The Revolution of Slim LED Display
The Rise of DesignByWarren: Redefining Windows and Doors in China
The Rise of Disposable Pod Systems: A Comprehensive Guide
The Rise of Disposable Vapes: A Simple, Flavorful, and Convenient Choice
The Rise of Korean Plastic Surgery: A Global Beauty Revolution
The Rise of Nangs Delivery in Sydney: Convenience, Quality, and Fast Service
The Rise of Outsourcing Companies: Transforming Business Operations
The Rise of Rechargeable Disposable Vapes: A Game-Changer in the Vaping World
The Rise of the Disposable Vape Pen: A Trend Worth Exploring
The Rise of WarrenGroup: Redefining Windows and Doors Manufacturing in China
The Rising Need for Armed Security Services in New York City: How USPASecurity is Redefining Protection
The Rising Trend of Korean Plastic Surgery: A Global Phenomenon
The Role of Disability Insurance Lawyers: Why Eric Buchanan and Associates Should Be Your Go-To Choice
The Safest Places in Mexico: A Guide to Secure and Beautiful Destinations
The Smarter Way to Shop: Style Meets Savings at Meshada Fashion
The Strategic Edge of Buying Instagram Followers: A Comprehensive Guide
The Strength and Style of Metal Roofs: Why PITCH Roofing is Florida’s Top Choice
The Timeless Allure of Marlboro Cigarettes: A Focus on Marlboro Red
The Timeless Fun of WOOSPIN’s Keno: Where Luck Meets Numbers
The Transformative Power of Lingam Massage in London: A Journey of Relaxation and Rejuvenation
The Transformative Power of Rejuran: A Comprehensive Guide
The Ultimate Experience with Your Photographer at StudioNewPortri: Crafting Timeless Memories
The Ultimate Game Guide: How Simple 2D Pixel Art Games are Captivating Players
The Ultimate Gift: Embrace the Power of Thoughtful Giving with Jescojes
The Ultimate Guide to a Paso Robles Wine Tour Experience
The Ultimate Guide to APKs: Unlocking the Power of MOD APKs with APKModJoy
The Ultimate Guide to B2B Marketplace Platforms: Revolutionizing Global Trade with GlobalTradeAxis
The Ultimate Guide to Bank Statement Conversion with StatementSheet: Efficiency, Security, and Accuracy
The Ultimate Guide to Breast Augmentation: Enhance Your Natural Beauty with MINE Plastic Surgery
The Ultimate Guide to Breast Lift: Enhancing Your Natural Shape with MINE Plastic Surgery
The Ultimate Guide to Buying Chevy Impala Auto Spare Parts: Affordable, Reliable, and Convenient
The Ultimate Guide to Buying Cigarettes Online: Your Path to Convenience with DreamCigs
The Ultimate Guide to Car Seat Safety: Laws, Best Practices, and Why It Matters for Your Child
The Ultimate Guide to Champagne: Discover the Bubbles, the Craft, and the Deals at Fine Wine Delivery
The Ultimate Guide to Cheap Cigarettes: Quality and Affordability
The Ultimate Guide to Choosing a Law Tutor: How CityLawTutors Bridges the Gap for Aspiring Legal Professionals
The Ultimate Guide to Choosing a Slidell Moving Company
The Ultimate Guide to Choosing Long Distance Movers in Ashland, MA
The Ultimate Guide to Choosing the Best Maid Agency: Why First Maid is Singapore’s Top Choice
The Ultimate Guide to Choosing the Perfect 84-Inch Window for Your Home
The Ultimate Guide to Choosing the Perfect Walk in Bath and Shower
The Ultimate Guide to Colored Contact Lenses: Transforming Your Look with ColoredContacts
The Ultimate Guide to Countertop Ice Makers: Convenience, Efficiency, and Versatility
The Ultimate Guide to Dresses: The Fashion Statement That Never Goes Out of Style
The Ultimate Guide to DTF Gang Sheets: Revolutionizing Custom Printing
The Ultimate Guide to Employee Holiday Software: Streamlining Time-Off Management with Workant
The Ultimate Guide to Finding the Best Dentist in Burlington: Your Path to a Healthy Smile
The Ultimate Guide to Gutter Installation in Phoenix: Protecting Your Home Year-Round
The Ultimate Guide to Man and Van Services in Northampton: Why TDN MAN&VAN Removals is Your Best Choice
The Ultimate Guide to Movers and Packers in Dubai: Shahi Movers
The Ultimate Guide to Neck Lift: Rejuvenate Your Appearance with MINE Plastic Surgery
The Ultimate Guide to Office Cleaning Services in Perth: Boosting Productivity and Well-being
The Ultimate Guide to Party Rentals: Elevating Your Event Experience
The Ultimate Guide to Plastic Cup Making Machines: Revolutionizing Manufacturing with TZMachinery
The Ultimate Guide to Removing Image Backgrounds for Free: Unleash Your Creativity with Skoshart
The Ultimate Guide to Shipping Stores in Canada: Why Beavership is Revolutionizing the Shipping Experience
The Ultimate Guide to Snowboard Goggles: Find Your Perfect Pair at Boardertown
The Ultimate Guide to Table & Chair Rentals: Transform Your Event with CPG Event Rentals
The Ultimate Guide to Tongits: Rules, Strategies, and How to Master the Game
The Ultimate Guide to Using Free Resume Templates in Microsoft Word
The Ultimate Guide to Washer Repair in Orange County: What You Need to Know
The Ultimate Guide to Wedding Invitations: Crafting the Perfect First Impression
The Ultimate Solution for Chicago's Rat Problem: Introducing the Sewer Assassin
The Versatile Plastic Turnover Box: A Game-Changer in Logistics and Agriculture
The WarrenSystem: Revolutionizing the Doors and Windows Industry
Tiết lộ Chiến thuật chơi tài xỉu uk88 bách chiến bách thắng
Tìm Hiểu Về Những Ứng Dụng Soi Cầu Lô Đề Hot Nhất
Time Tracking Software: Revolutionizing Workforce Management
Tips for Connecting a New Car Radio
Toko Terdekat: Kemudahan Belanja di Era Digital
Top 5 Remote Job Platforms for Indians in 2024
Top Influencer in Crypto: Shaping the Future of Digital Currency Conversations
Toronto Houses for Sale: Your Comprehensive Guide to Finding the Perfect Home
Traffic Management Companies Melbourne: A Vital Solution for Urban Mobility
Traffic Management Companies Melbourne: Ensuring Safety and Efficiency on the Roads
Transform Your Cooking Experience: How the Right Kitchen Tools and Cookware Can Make Meal Prep a Joy
Transform Your Hair Game: Discover the Ultimate Electrical Styling Tools at Holy Grail Haircare
Transform Your Space with an 8 ft Patio Glass Door: A Complete Guide
Transforming Fashion Shopping: How Meshada Redefines Style and Savings
Transforming Housing Society Management with Billing Software: A Step Toward Efficiency and Transparency
Transforming Spaces with Handmade Cabinet Knobs: The Perfect Finishing Touch
Transforming Spaces: The Ultimate Guide to Kitchen Renovation with Calista Homes
Transforming Workspaces: The Ultimate Guide to Office Cleaning Services in Perth
Transforming Your Home: The Art of Kitchen Remodeling with Calista Homes
Trasteros en Barcelona: La Solución Perfecta para el Espacio en la Ciudad
Tree Stump Removal Service in Buckhead: A Vital Step for Your Lawn’s Health and Beauty
Trivia Near Me Tonight: Unleashing the Fun with Great Big Trivia
Trivia Team Building in Sarasota: A Fun and Effective Way to Strengthen Your Team
Trump Tower Jeddah: A Monument of Luxury and Modern Living
Trusted Online Casino Malaysia: A Guide to Safe and Exciting Gaming with OCS8
Tummy Tuck: Solusi Kecantikan dan Kepercayaan Diri Anda
Uber Inspection Made Easy: The Insve Advantage
Uncorking the Beauty of Paso Robles: Your Ultimate Wine Tour Experience
Understanding 3 Gallon Nursery Pot Size: A Comprehensive Guide
Understanding and Addressing the Issue of a Double Chin: A Guide to Treatment Options
Understanding Apostille Services: A Guide by Notary DC Maryland Virginia
Understanding Blocked Drainage: Causes, Consequences, and Solutions
Understanding Breast Reduction Surgery: A Comprehensive Guide
Understanding Concrete Driveways in Perth: Prices, Factors, and How to Save
Understanding Data Recovery: A Guide to Safeguarding Your Valuable Digital Assets
Understanding Fat Transfer Breast Augmentation: A Natural Approach to Enhancing Your Body
Understanding Queen Anne Gutter Leaning: A Crucial Concern for Homeowners in Seattle
Understanding Sodium Ion Battery Prices: The Future of Energy Storage
Understanding Soma Medication: Uses, Benefits, and Considerations
Understanding Texas Lobbying Firms: The Role of Advocacy in the Lone Star State
Understanding the Aviation English Language Proficiency Test: A Key to Career Advancement in Aviation
Understanding the Types of Chinese Swords: A Journey Through History and Craftsmanship
Understanding Tummy Tuck: A Path to a Healthier, More Confident You
Understanding Website Cost: A Comprehensive Guide for Small Businesses
Understanding What a Trading Education Company Does: A Deep Dive into Certus Trading
Unforgettable Event Experiences: Discover the Best Catering Services in Kuala Lumpur
Unleash Your True Style with Huffer and Backdoor: The Ultimate Surf, Skate, and Streetwear Experience
Unleashing the Power of Rust Hosting with Keks Host
Unleashing the Power of Tampa Team Bonding: Elevate Your Next Corporate Event
Unlock Your Best Smile: Discover Cosmetic Dentistry Near You
Unlock Your Gaming Potential with the Stand Mod Menu: Elevating the GTA V Experience
Unlock Your Website's Potential with a Buy Backlinks Package
Unlocking Customer Insights with the Kano Model Template: A Comprehensive Guide
Unlocking Efficiency: The Power of Printable Chart Templates
Unlocking Efficiency: Why Comp-Service is Your Go-To for Computer Repair
Unlocking Online Success with SpeedyIndex: Accelerate Your Website’s Indexing Journey
Unlocking Online Success: The Power of SEO Services by Arfadia
Unlocking Opportunities: A Guide to Security Officer Vacancies with Collins Career Solutions
Unlocking Potential: Insights from WarrenExpert in the Window and Door Industry
Unlocking Radiance: Discovering the Best Retinol Body Lotion
Unlocking Success with Affiliate Marketing: A Guide for Financial and Personal Growth
Unlocking the Future of Blockchain Development: The Power of Reploy
Unlocking the Future of Roblox Gaming: The Now.gg Advantage
Unlocking the Mystery of Apostille Services Near Me
Unlocking the Path to Inner Peace: 200 Hour Yoga Teacher Training in Rishikesh
Unlocking the Perfect Proposal: Creative Ideas & Essential Tips for Choosing the Ideal Engagement Ring
Unlocking the Power of a Defined Jawline: How MINE Plastic Surgery Can Enhance Your Natural Beauty
Unlocking the Power of APK Downloaders: A Guide to APKPhat.net
Unlocking the Power of Business Consultancy: How Rezex Accountants Helps You Turn Your Plans Into Reality
Unlocking the Power of Comp-Service: Elevating Business Performance
Unlocking the Power of MLS Vaughan: How Homsy is Revolutionizing Real Estate
Unlocking the Power of Online Business: A Path to Financial and Personal Growth with Vantage Quest
Unlocking the Power of Puzzles: A Journey into Cognitive Fitness
Unlocking the Power of the 24Hour Challenge: A Unique Journey of Growth and Discovery
Unlocking the Secrets of Advanced Snail 96 Mucin Serum: Your Pathway to Radiant, Youthful Skin
Unlocking the Secrets of Relaxation: The Transformative Power of Lingam Massage in West London
Unlocking the Secrets to a Youthful Look: Everything You Need to Know About Facelifts
Unlocking the Secrets to Exceptional Garden Design: A Guide to Creating Beautiful Outdoor Spaces
Unlocking the Thrills of Slot Free Credit at PG Slot 2
Unlocking Your Body's Potential: The Power of Physio in Abu Dhabi
Unlocking Your Path to Academic Success with On Thi Dgnl: The Ultimate Resource for Entrance Exams and School Competency Assessments
Unpacking the Buzz: Backpack Boyz Carts
Unraveling the Pulse of EDM: The Beat That Unites Us All
Unveiling Radiance: The Power of Dr Pen by Labode Beauty
Unveiling the Future of Search: Neurodynamics and SpeedyIndex Research
Unveiling the Magic of Dresses: Embrace Chic Elegance with Trending Chic
Unveiling the Path of the Spiritual Guru: Insights from Adrian Gabriel Dumitru
Unveiling the Power of the NSFW Story Generator: A New Frontier in Digital Creativity
Unveiling the Secrets of the Korean Facelift: A Modern Approach to Youthful Skin
Unveiling the World of SDM Sugar Daddy Dating: A Luxurious Journey Awaits
Urgente Stomatologice Copii Cluj: Cum Să Gestionăm Corect Situațiile de Urgență Dentară la Copii
Usługi Pogrzebowe Warszawa Ochota: Zaufaj Nadziei w Trudnych Chwilach
Verde Casino: Laimz – Jūsu Ceļš Uz Neaizmirstamām Spēļu Pieredzēm
Videncia en Madrid: Todo lo que Necesitas Saber Sobre las Consultas de Futuro
Vientre en Alquiler: Una Mirada Profunda al Futuro de la Reproducción Asistida
VIP Indicator Review: Unveiling the Secrets of Successful Trading
Vortex Plumbing Inc.: The Trusted Plumbing Company in King County, WA
Warren Storefront Windows: Crafting Excellence in Design and Quality
Warren Window Store: Revolutionizing Home Design with Quality Aluminum Windows and Doors
Warren Window: Revolutionizing the Chinese Window and Door Industry
Warren Windows and Doors: Elevating Homes with Precision and Innovation
WarrenExpert: Elevating Your Windows and Doors Experience
Waterproof Flood Lights | Illuminate Africa with Confidence
WDMA Windows and Doors: Shaping the Future of Residential and Commercial Spaces
Website Designer Cincinnati: Transform Your Online Presence
Website Google Index Checker: Boosting Your Site's Visibility with SpeedyIndex
Weight Sensor Load Cell: Revolutionizing Industrial Measurement for a Smarter Future
Westinghouse Spare Parts NZ: Keeping Your Appliances Running Smoothly with ApplianceSpares
What It Means to Accomplish Goals and Objectives in Today’s Business Environment
What Makes a Leader Truly Impactful and Why Sustainability Matters
Why a 16 x 8 ft Insulated Garage Door is a Smart Investment for Your Home
Why Choosing the Right Realtor is Key to a Seamless Home Buying and Selling Experience
Why Is My Dishwasher Not Draining? Common Causes and Solutions
Why lifepo4 battery supplier Aren’t Unnecessary
Why TDN Man and Van Removals is Kettering’s Best Choice for Your Next Move
Why You Need a Digital Business Card?
Why You Need a Pensacola Personal Injury Attorney: Expert Guidance from FenimoreInjuryLaw
Why You Should Buy Wana Gummies Online: The Ultimate Guide to Cannabis-Infused Edibles
Why You Should Choose a Real Estate Agent in Toronto with Homsy
Why You Should Visit DreamCigs.com: Your Ultimate Destination for Premium Cigarettes and Tobacco Products
Wigs with Bangs: Elevate Your Style with Confidence
Winning Plus: Unlocking Limitless Gaming Potential at WP Casino
Woospin Casino: A Comprehensive Guide to Gaming Excellence
Zeus Slot: Menyelami Sensasi Jackpot Maksimal di MACAU123
Z-Library: The World’s Largest Ebook Library and Its Impact on Digital Learning
Въведение в онлайн казината: Какво трябва да знаете
Как да управлявате банкрола си при игра на казино игри
Как справиться с проблемами сливания воды в стиральных машинах: Советы по ремонту от компании fixbc
Най-добрите варианти на онлайн рулетка за 2024 година
Най-популярните казино игри с бонус нива
بت 212: تجربهای متفاوت در دنیای شرطبندی آنلاین
بلیط هواپیما تهران استانبول: انتخاب هوشمندانه برای سفری بینظیر
بلیط هواپیما موج زمزم: تجربهای فراموشنشدنی در صنعت گردشگری
تعاونية تاج: جودة العسل والزيوت الطبيعية في عالمنا الحديث
แทงบอลออนไลน์: ประสบการณ์การเดิมพันที่ดีที่สุดกับ UFABET
การสร้างธุรกิจที่เติบโตด้วยกลยุทธ์ที่พิสูจน์แล้ว
บัญชี Facebook: ทำความเข้าใจและการใช้ประโยชน์ในการตลาดออนไลน์
หนังโป๊: การสำรวจโลกแห่งสื่อและผลกระทบทางสังคม
チタニウムリフトで若々しい肌を実現する方法
フェイスリフト:肌の若返りと美しさを取り戻す方法
꽁머니와 안전한 배팅을 위한 갬빗 먹튀검증 커뮤니티의 역할
리쥬란: 피부 재생과 탄력 회복의 새로운 시대
먹튀검증: 안전한 토토 사이트 선택의 첫걸음
먹튀폴리스: 먹튀사이트의 위험성과 예방을 위한 필수 정보
무료슬롯: 온라인 카지노 게임의 새로운 즐거움
무료슬롯: 온라인 카지노의 매력을 더욱 높이다
송파가라오케: 윈가라오케의 최신 정보와 예약 가이드
시술 부작용: 주의해야 할 사항과 이해하기
안면거상: 마인성형외과의 새로운 시대
안전놀이터: 어린이들의 안전을 보장하는 첫 번째 단계
온라인 카지노사이트 선택의 모든 것: 카지노프렌즈777의 가이드
온라인카지노: 안전하고 신뢰할 수 있는 사이트 선택의 중요성
완벽한 턱선을 위한 선택: 마인성형외과의 노하우
윈가라오케: 송파구 잠실 최고의 가라오케 경험
인터넷 슬롯 머신 사이트: 새로운 엔터테인먼트 세계로의 초대
잠실 윈가라오케: 송파구에서 최고의 노래방 경험을 제공하는 곳
잠실 윈가라오케: 최고의 노래방 경험을 제공하는 장소
주소모음: 링크집에서 제공하는 편리한 인터넷 서비스 활용법
주소야: 디지털 시대의 필수 도구, 링크 관리의 혁신
중고트럭: 스마트한 선택으로 경제적인 운송 솔루션 찾기
二重顎の悩みを解消!マイン皮膚科で理想のフェイスラインを手に入れる方法
카지노사이트를 선택할 때, 왜 카지노프렌즈인가?
双下巴问题的解决方案与理想下颌线条的塑造
킹카지노: 온라인 카지노의 선두주자
턱살 제거: 효과적인 방법과 마인성형외과의 맞춤형 해결책
토토사이트: 안전한 선택의 중요성
垂れ乳の原因と解決策:マイン皮膚科からのアプローチ
함몰유두: 원인, 치료 방법 및 마인성형외과의 접근법
拉皮手术:恢复年轻与美丽的突破
美版超声刀:麦恩整形外科的革命性皮肤治疗
翻譯社:AI與遠端作業如何重塑翻譯產業
自体脂肪丰胸:丰满与安全的完美结合
臺北輕鬆推薦:臺北輕鬆會館
豊胸手術とマイン美容外科:美しい体型を手に入れるために
隆胸手术:麦恩整形外科为您带来的全新体验
面部拉皮:重塑青春,恢复紧致与轮廓
Public
Exotic THCA Flower: A Journey into the World of Rare Cannabis Delights
Unveiling Your Computer's Past: How to Check Computer Usage History
✨ 건강한 삶의 동반자, 마사지: 몸과 마음을 위한 최고의 선물 🎁
마즈지티비의 성장과 한국 미디어 산업의 미래
Introducing Paintbuddy&CO, Sydney’s Newest Painting Service!
10 List of Online Slot Providers Often Called Gacor Win88 Sites
2024년 최신 슬롯사이트 순위 TOP10
2024년 최신 슬롯사이트 순위 TOP10 소개
Accompagner son enfant hypersensible : un voyage au cœur des émotions
Alasan Bermain Demo Wild Bounty Showdown Pg Disini
Aménager un petit espace : astuces gain de place pour une maison fonctionnelle et stylée
BGA Assembly: Ensuring Quality and Reliability
Bossware: The Invisible Eye Tracking Remote Workers
Canberra Commercial Real Estate: Strategic Investments
Caterpillar Zeppelin companies actively circumventing their own sanctions against Russia
Choosing Skull Wall Art For Your Home
Comprehensive Moving Services in Slidell: Pack Dat & Geaux Movers
Cybersecurity Las Vegas: Protecting the Gaming Industry from Cyber Threats
Cybersecurity Services Las Vegas: Protect Your Digital Assets
Democratizing Data: The Power of Self-Service Financial Reporting
Discover Trendy Home Decor Accessories at Our Online Store
Discovering the Benefits of Bono Hair
Elevate Your TikTok Game with Our Engagement Boost
Employee Monitoring Insights: Navigating the Modern Workplace
Empowering Communities and Women: The Inspiring Story of a Visionary Social Entrepreneur
Enhancing Perimeter Security with Certified Crash-Rated Bollards
Enhancing Your Digital Presence with Expert Web Design Services
Expert Tips for Dryer Duct Cleaning Los Angeles
Explore the Thrills of Online Gaming with DJTOGEL
Exploring Garlics.com: Your One-Stop Shop for High-Quality Chinese Garlic
Exploring the Smart LED TV Market in Pakistan: Prices and Trends
Facebook SEO: Your Secret Weapon for Organic Reach in a Sea of Content
Gear Up for HackTech: The Ultimate Tech Gathering in Cyprus
Going Green with Battery Inverters: Reducing Your Carbon Footprint
Harnessing the Power of Internal Communication Tools for Business Success
Harnessing the Power of Website Design in Charlotte
Have you ever thought about the impact of product packaging design?
Hebrew to English Bible: Discovering the Divine Message in Translation
High Ticket Affiliate Marketing for Beginners: Your Gateway to Massive Earnings
How Clear Aligners Work: A Step-by-Step Guide
How to Be a Successful Investor: Lessons from Top Performers
How to Be an Effective Leader in Today's Real Estate Industry
How to Choose the Best Ladies Bag Wholesale From DHgate
How to Choose the Best Residential Movers in St. Louis, MO?
How to Create Eco-Friendly Product Packaging for Your Custom Product Packaging Boxes
How to Effectively Market Organizations
How to Make the Best Use of Speed Bump Structures
How to Open Glass Bottle Without Opener
How Topnotch SEO NZ Can Help You Achieve Web Visibility
iGaming Advertising: Desktop vs. Mobile - Where Should You Focus Your Efforts?
Impact Electrical - The Best Electrician on the North Shore!
InarasCases: More Than Just a Pretty Face - It's a Community!
iw777 APK: Unleash the Fun on Your Android Device
Learn about AgilityPortal | The Leading Staff Communication App
Mastering The Craft: Exploring The World Of Culinary Damascus Knives
Maximize Comfort and Efficiency with China WDMA's Energy-Efficient Casement Windows
Maximize Your ROI with China PCBA's PCB Assembly Services
Maximizing ROI: Leveraging PropellerAds for Effective Performance Marketing
Michelin Circumvents Sanctions to Continue Tire Sales in Russia
Mid-Century Armchairs: The Epitome of Timeless Style and Comfort
Mortgage Broker Belfast: Helping You Find the Perfect Home Loan
Navigating the Complexities of Modern Leadership: A Guide for Today's Business Environment
Oil Blocker Barrier Boom
Ottawa Bloggers: Your Ultimate Guide to the Capital City
Packaging Powerhouse: The Top Reasons to Opt for Cardboard Boxes
Pest Control Glass House Mountains: Free Inspections to Assess Your Needs
Private Detective Services in Barcelona
Protecting Your Home and Business from Unwanted Guests
Revolutionize Your Business with LG Networks, Inc
Revolutionizing Industrial Efficiency with Endress Hauser MB05C5043BB
Sådan starter du et tømrerfirma
Smart Videos: The AI Advantage
Stavmixer Bäst i Test: En Djupgående Granskning
SweetNight: A Great Mattress for A Restful Sleep
The Allure of Real Estate Investment in Istanbul: Where History Meets Modern Luxury
The Art and Science of SEO: Insights from Top SEOs
The Benefits of Learning Quran Online with Experienced Teachers
The Benefits of Seashore Rubber and Rent One Hose
The Elegance and Functionality of Lounge Radiators
The Emergence of Level 2 EV Chargers: Why Evcstar Leads the Pack
The Future of Cannabinoid-Based Treatment: Buy K2 Paper for Sale
The Importance of Eco Safe Cleaning: Why Chimney Sweep Services Near Me Are Essential in Los Angeles
The Importance of Logistics Technology for Businesses
The Importance of SEO and Website Traffic
The Intricacies of Baccarat: A Guide for Online Casino Players
The Magic of Custom Bobbleheads: A Unique Gift for Your Loved Ones
the product packaging Blog/Forum
the product packaging Blog/Forum
the product packaging Locations
the product packaging Web Content
the product packaging Web Content
The Revolutionary Dough Kneading Machine: Transforming Food Production
The Rise of SSD External Hard Drives: Revolutionizing Portable Storage
Thunder Laser: The Revolutionary Laser Cutting Technology
Time is Precious: Invest in Yourself with Home Massage
Top FUT 23 Players With The Highest Dribbling Attribute
Top Web Designing Softwares
Transform Your Packaging Strategy with the Dynamic Duo: Chipboard Pads and Mailing Boxes
Types of Custom Bolts and Nuts
Understanding Tariffs and Duties: How Customs Brokers Help Reduce Costs
Unlock the Full Potential of Your Home: The Benefits of Loft Conversions in Rotherham
Unwind in Your Hotel: The Luxury of Business Trip Massage Houses
Upgrade Your Philadelphia Network: Professional Cabling Solutions
Valg af den rigtige juridiske struktur for dit rengøringsfirma
ViasionPCB: Your Trusted Partner for High-Quality, Low to Medium Volume PCBs
Ways to come up with naming
What is CPC? Demystifying Cost-Per-Click Advertising
What It Takes to Be a Leader in Community Building: Data-Driven Decision Making
What It Takes to Be a Leader in Community Building: Data-Driven Decision Making
What It Takes to Be a Leader in Senior Services
Yabancılarla Görüntülü Sohbet: Dijital Çağda İletişimin Yeni Yüzü
Year-Round Pest Protection in Beerwah: Choose Luke's Termite and Pest Control
걸스티비: 여성의 목소리를 세상에 전달하는 새로운 미디어 플랫폼
러시아 마사지의 비밀
러시아 출장 마사지의 매력
레플리카: 고품질, 저렴한 가격, 완벽한 선택? 진실과 오해를 풀다
레플리카: 나만의 럭셔리, 나만의 스타일을 완성하다
리니지 프리서버 스킬: 스킬 마스터 가이드 📚🔥
마징가티비: 스포츠 중계의 새로운 지평
마징가티비가 변화시킨 시청자와 크리에이터의 관계
먹튀검증 업체 순위 TOP 10: 믿을 수 있는 검증 사이트는?
먹튀검증, 안전과 신뢰를 최우선으로 생각합니다!
먹튀검증, 이제 안전하게 베팅하고 즐겨라!
먹튀검증업체: 안전한 배팅을 위한 필수 가이드
먹튀검증의 중요성: 안전한 스포츠 베팅의 시작
먹튀사이트: 안전한 배팅을 위한 필수 검증
메이저사이트, 안전하고 즐거운 베팅을 위한 선택
모바일 바카라사이트: 언제 어디서든 즐기는 카지노 게임
몸을 힐링하는 최고의 방법, 마사지의 진정한 가치
바카라 사이트 FAQ: 궁금증을 해결해 드립니다
바카라 사이트 FAQ: 궁금증을 해결해 드립니다
바카라사이트 라이브 게임: 실제 딜러와 함께 즐기는 생생한 경험
바카라사이트 최고의 사이트는 어디일까? 신중한 선택을 위한 가이드
바카라사이트: ⚡️ 스피드 바카라로 즐기는 쾌속 질주! ⏱️ 숨 막히는 속도전에 도전하세요!
바카라사이트: 숙련된 딜러와 함께하는 실감나는 게임
바카라사이트: 집에서 즐기는 라스베가스, 실제 카지노보다 더 생생하게!
바카라사이트: 카지노에서 바카라 게임을 즐기는 방법
스웨디시 테라피, 건강과 행복을 위한 최고의 선택: 몸과 마음의 완벽한 조화
스포츠사이트 프로모션: 놓치면 아까운 다양한 혜택!
슬롯사이트: 한국 카지노 슬롯 게임의 매력과 이점
안전공원주소: 스포츠 베팅의 새로운 표준을 세우다
안전공원주소를 통해 만나는 프리미엄 베팅 서비스
안전하고 흥미진진한 온라인 홀덤: 레볼루션홀덤의 매력
안전한 스포츠 베팅: 먹튀검증의 중요성과 방법
에볼루션 게임: 한국 플레이어를 위한 최고의 선택
에볼루션카지노: 잊지 못할 게임 경험을 선사하는 마법
에볼루션카지노: 잊지 못할 게임 경험을 선사하는 마법
에프페시아, 탈모약 효과 극대화 하는 방법! 🚀
에프페시아: 탈모 치료의 새로운 패러다임
인천 쓰리노: 특별한 순간을 위한 프리미엄 노래방, 그 이상의 가치
인천 쓰리노: 특별한 순간을 위한 프리미엄 노래방, 그 이상의 가치
최고의 바카라사이트를 선택하는 방법
카지노 바카라 게임의 매력과 장점
카지노 중독 치료: 최신 연구와 방법
카지노 커뮤니티 멤버들의 게임 중 미신과 행운의 징조: 승리를 부르는 마법의 주문?
카지노 커뮤니티: 궁금증 해결하고 더욱 스마트하게 즐기세요!
카지노 커뮤니티와 함께하는 행복한 게임 생활: 즐거움과 책임감의 조화
카지노 커뮤니티와 함께하는 흥미진진한 카지노 여정
토토 커뮤니티, 배팅 고수들의 비밀 노트: 승률을 높이는 숨겨진 노하우 대공개!
토토사이트 먹튀검증커뮤니티: 베터들의 정보 공유와 안전한 베팅을 위한 길잡이
토토사이트 프로모션: 놓치면 아까운 다양한 혜택! 🎁
토토사이트, 고객 만족을 위한 최상의 서비스: 차별화된 경험으로 승부한다
토토사이트, 고객 만족을 위한 최상의 서비스: 차별화된 경험으로 승부한다
투데이서버, 리니지 프리서버의 끝판왕
핀페시아 가격 비교: 최저가는 어디?
핀페시아 중단, 어떤 일이 일어날까요?
핀페시아 직구 - 탈모 치료의 새로운 희망
핀페시아 후기 이벤트: 솔직한 후기 남기고 경품 받으세요! 🎁
핀페시아, 더 이상 탈모로 스트레스 받지 마세요!
핀페시아, 효과적인 탈모 치료 방법
핀페시아직구, 자신감 회복 프로젝트
한국 카지노의 매력: 흥미진진한 게임과 독특한 문화의 만남
If this is your company, CONTACT US to activate Packbase™ software to build your portal.
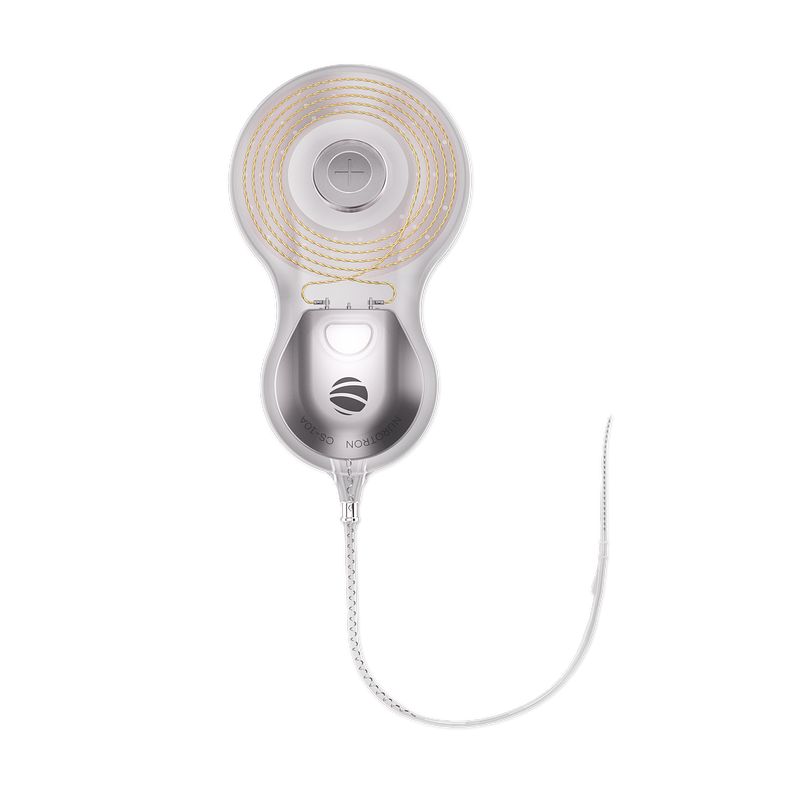
Did you know that approximately 466 million people worldwide experience disabling hearing loss, according to the World Health Organization? Among them, a significant number are candidates for bilateral hearing loss implants, which have revolutionized auditory rehabilitation. As we delve into this topic, it is crucial to understand not only the medical implications but also the legal and regulatory frameworks governing these devices.
The Framework Surrounding Bilateral Hearing Loss Implants
Bilateral hearing loss implants are sophisticated medical devices designed to improve auditory perception in individuals with severe to profound hearing impairment. Their legal attributes stem from stringent regulations aimed at ensuring safety and efficacy. In many jurisdictions, these implants must comply with health standards set forth by governmental bodies such as the FDA in the United States or CE marking in Europe. Furthermore, they fall under specific Health and Safety Regulations that mandate rigorous testing before market approval, thereby safeguarding patient welfare.
Nurotron’s Compliance with Health and Safety Regulations
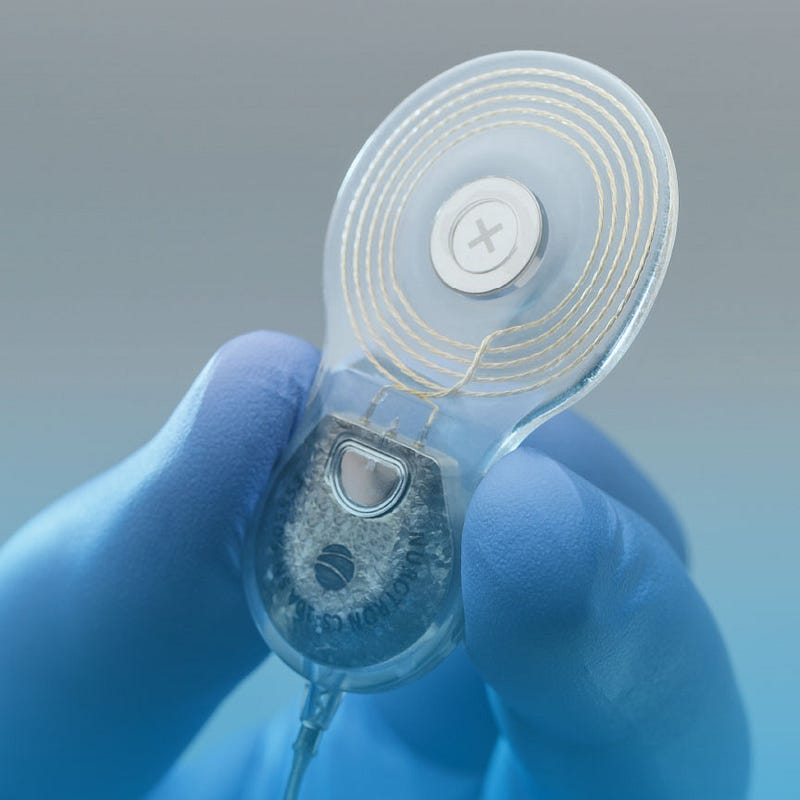
Nurotron Technology Co., Ltd., a prominent player in the field of cochlear implant technology, exemplifies adherence to Health and Safety Regulations through its commitment to quality assurance processes. Nurotron ensures that all products undergo extensive clinical trials before receiving regulatory clearance. Additionally, their manufacturing practices align with ISO standards for medical devices — further reinforcing their dedication to producing safe and effective solutions for patients suffering from bilateral hearing loss.
Cochlear Implants for Kids: Key Features Under Health and Safety Regulations
- Age-Specific Design: Cochlear implants designed for children must meet unique developmental needs; hence they undergo specialized testing protocols.
- Safety Standards: These devices adhere strictly to pediatric safety guidelines established by health authorities globally.
- User-Friendly Interfaces: Devices intended for younger users often incorporate intuitive controls tailored for ease of use among children.
- Long-term Monitoring Requirements: Regulatory frameworks necessitate ongoing assessments post-implantation to monitor device performance over time.
- Pediatric Clinical Trials: Before approval, cochlear implants specifically made for kids require participation in dedicated clinical studies focusing on long-term outcomes related to speech development and social integration.
A Conclusive Overview
The landscape surrounding bilateral hearing loss implants is characterized by comprehensive legal regulations focused on ensuring patient safety while promoting technological advancement. From manufacturers like Nurotron adhering rigorously to health standards, particularly concerning pediatric applications of cochlear implants — these measures collectively enhance public trust in such life-changing technologies. Understanding these regulatory aspects is essential as we navigate an increasingly complex healthcare environment where innovation meets regulation head-on.
Click cochlear implant for kid.







.png)






.jpeg)
