If this is your company, CONTACT US to activate Packbase™ software to build your portal.
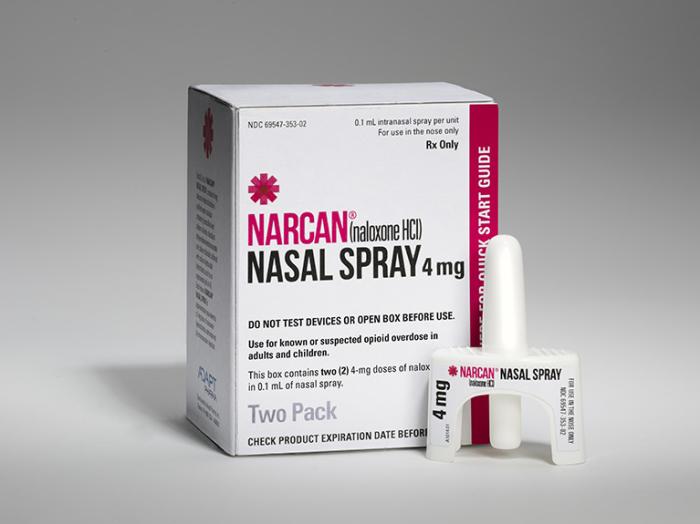

Aptar Pharma has announced that it has provided the delivery system and regulatory support for Adapt Pharma’s Narcan® which is the first U.S. FDA-approved nasally administered, needle-free, ready-to use medication that can stop or reverse the effects of an opioid overdose.
Working together to achieve combination drug–device product compliance and regulatory approval Narcan contains naloxone which is an opioid antagonist. It is marketed in the U.S. for emergency treatment of known or suspected opioid overdose.
“The U.S is facing an unprecedented opioid overdose epidemic. By making Narcan Nasal Spray available, we hope to increase everyone’s access to emergency treatments across the U.S.,” said Seamus Mulligan, Chairman and CEO of Adapt Pharma. Aptar Pharma has brought to the partnership its unique experience in nasal drug delivery and regulatory expertise in relation to combination drug-device products successfully supporting Adapt Pharma’s new drug application.
“Aptar Pharma leads the industry in nasal drug delivery expertise. We have collaborated closely with Adapt Pharma to ensure the rapid approval of this potentially life-saving product using our Unit-Dose System (UDS). The regulations and requirements for market approvals are rapidly evolving and customers must seek out trusted partners like Aptar Pharma. We are continually investing in resources and capabilities to support our customers in reaching the market quickly,” said Salim Haffar, President of Aptar Pharma.
Continuous expansion of non-invasive nasal sprays for systemic drug delivery estimated at over $2 billion in 2015, the world market for intra-nasal spray systemic drug delivery has experienced strong growth in the last few years. The list of therapies successfully treated via the nasal route continues to grow from the traditional areas of migraine, osteoporosis and endometriosis, to now include: post-operative pain (ketorolac), cancer pain (fentanyl), prostate cancer, urinary incontinence, anemia and vaccinations. UDS: the proven technology platform for unit-dose liquid drug delivery Aptar Pharma’s UDS is the preferred liquid spray drug delivery technology platform when dose accuracy and ease of administration are critical. The single-shot UDS system’s primeless feature combined with 360° orientation offer the patient or the caregiver unique ready-to-use convenience.


.jpg)



.jpg)
.png)
.jpg)


 copy.jpg)


.jpg)
.jpg)























.jpg)


