If this is your company, CONTACT US to activate Packbase™ software to build your portal.
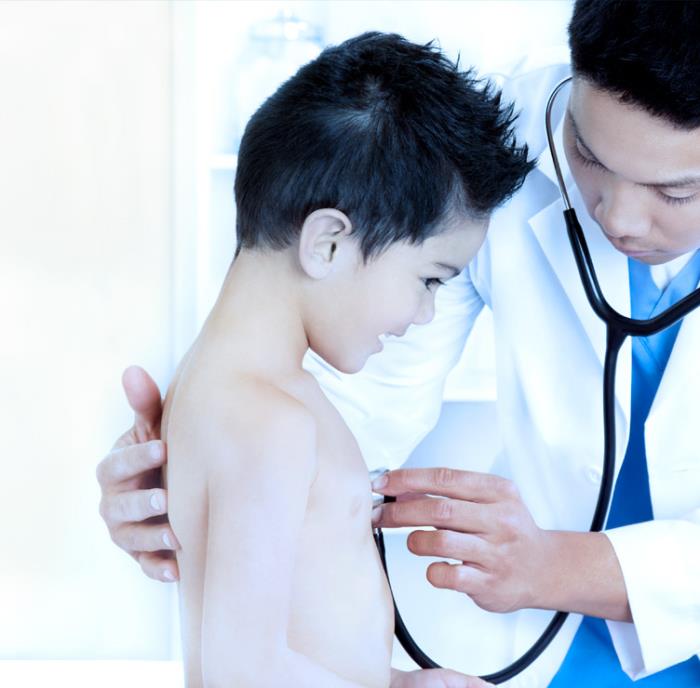

Aptar Pharma, the leader in respiratory drug delivery systems, is proud to announce its co- organization and diamond sponsorship of the Respiratory Drug Delivery (RDD® ) Europe 2019 scientific conference, connecting pulmonary and nasal drug delivery experts from around the world in Estoril, Portugal from May 7-10, 2019.
One of the largest respiratory health conferences worldwide, RDD Europe 2019 is a premium, interactive three-day conference offering a prestigious program that includes a plenary lecture and seven plenary sessions hosted by industry experts - including a U.S. FDA speaker - who will share their perspectives and latest accomplishments in pulmonary and nasal drug delivery.
This international symposium also offers delegates the latest insights into issues facing the global inhalation community, and numerous business networking opportunities.
As part of their Diamond Sponsorship, Aptar Pharma will lead three interactive workshops dedicated to connected health. Entitled “The Future of Connected Medicines: Promise and Potential Pitfalls”, these sessions will take place on Wednesday, May 8 and will be presented by Sai Shankar Director, Business Development - Connected Devices at Aptar Pharma along with David Van Sickle, Co-founder and CEO at Propeller Health.
Reaffirming their respiratory expertise, Aptar Pharma will also present two scientific posters during RDD Europe 2019, co-presented by Dr. Gerallt Williams, Director Scientific Affairs, Aptar Pharma:
• “Human Factor studies for unit-dose nasal sprays – a case study”, co- presented by G. Williams, B. Grosjean, D. Chopard, P. Schwartz, J. Cabiddu
• “Investigation of leachables from pMDIs containing HFA 152a”, co-presented by S. Sarrailh, A. Piquenot, O. Michelet, G. Williams.
In addition to co-organizing the conference, Aptar Pharma will showcase their extensive portfolio of MDIs, DPIs and BAIs, including the growing range of connected devices, at an interactive Exhibit Table.
Available as either add-on or fully integrated, Aptar’s c-devices can deliver improved patient health outcomes through better adherence. Also, discover the portfolio of active packaging solutions from Aptar CSP Technologies, now part of the Aptar family, which can help optimize your drug product’s packaging environment.
As Platinum Sponsor, Aptar Pharma will be hosting the Gala Dinner at RDD Europe 2019, which will take place on Thursday May 9 at Sud Lisboa, located on the banks of the Tagus River in Lisbon. Gael Touya, President of Aptar Pharma, will be on hand to welcome guests to this much-anticipated event, which combines fine-cuisine, dancing and networking to cap off the conference.
Register now at https://www.rddonline.com/rddeurope2019 for RDD Europe 2019 – a must-attend conference for companies involved in research, development, testing and marketing of medicines, as well as devices and services associated with pulmonary or nasal products.



.jpg)



.jpg)
.png)
.jpg)


 copy.jpg)


.jpg)
.jpg)























.jpg)


