If this is your company, CONTACT US to activate Packbase™ software to build your portal.
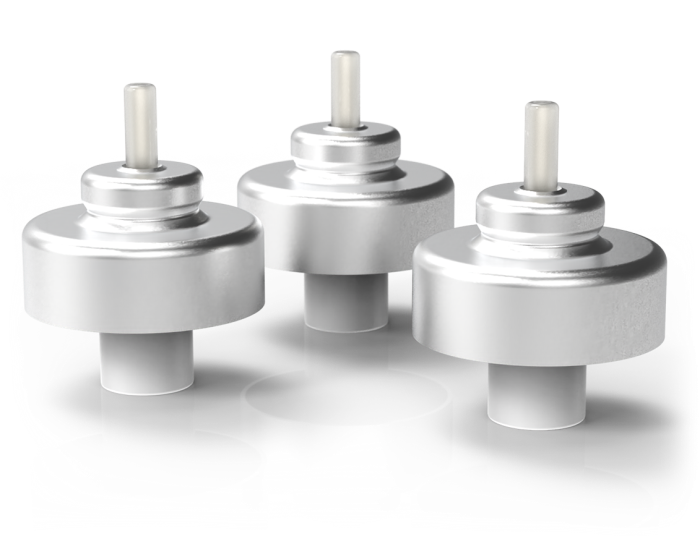

MDI metering valve for inverted use
Overview | |
| Reference - Sub | Metering valve for inverted use |
|---|---|
| Description - Long MDI metering valve for inverted use, with 20 mm mounting cup. A collar for the complete emptying of the contents is employed. Suitable for HFA & CFC-propelled formulations and double stage filling over the internal gaskets. | |
| Packaging Type | Aerosols |
| Packaging Sub-Type | Aerosol Valves |
| Market - Major | Beauty, Personal Care. Health |
| Market - Segment | Cosmetics. Personal Care. Fragrances. Pharmaceuticals |
| Market - End Use | Nails. Hair. Hair Care. Female Fragrances. Male Fragrances. Illness Prevention. First Aid |
| Certifications | Laboratory, Testing, Certification. ISO Certifications. FDA Approved |
Characteristics | |
| Materials | Plastic. Metal |
|---|---|
| Feature Notes • FDA-approved materials for plastic components | |
Dimensions | |
| Diameter | 20 mm |
|---|---|
Dispensing | |
| Dispensing/Dosing Volume | 50 µl |
|---|---|
Overview | |
| Reference - Sub | Metering valve for inverted use |
|---|---|
| Description - Long MDI metering valve for inverted use, with 20 mm mounting cup. A collar for the complete emptying of the contents is employed. Suitable for HFA & CFC-propelled formulations and double stage filling over the internal gaskets. | |
| Packaging Type | Aerosols |
| Packaging Sub-Type | Aerosol Valves |
| Market - Major | Beauty, Personal Care. Health |
| Market - Segment | Cosmetics. Personal Care. Fragrances. Pharmaceuticals |
| Market - End Use | Nails. Hair. Hair Care. Female Fragrances. Male Fragrances. Illness Prevention. First Aid |
| Certifications | Laboratory, Testing, Certification. ISO Certifications. FDA Approved |
Characteristics | |
| Materials | Plastic. Metal |
|---|---|
| Feature Notes • FDA-approved materials for plastic components | |
Dimensions | |
| Diameter | 20 mm |
|---|---|
Dispensing | |
| Dispensing/Dosing Volume | 65 µl |
|---|---|
Overview | |
| Reference - Sub | Metering valve for inverted use |
|---|---|
| Description - Long MDI metering valve for inverted use, with 20 mm mounting cup. A collar for the complete emptying of the contents is employed. Suitable for HFA & CFC-propelled formulations and double stage filling over the internal gaskets. | |
| Packaging Type | Aerosols |
| Packaging Sub-Type | Aerosol Valves |
| Market - Major | Beauty, Personal Care. Health |
| Market - Segment | Cosmetics. Personal Care. Fragrances. Pharmaceuticals |
| Market - End Use | Nails. Hair. Hair Care. Female Fragrances. Male Fragrances. Illness Prevention. First Aid |
| Certifications | Laboratory, Testing, Certification. ISO Certifications. FDA Approved |
Characteristics | |
| Materials | Plastic. Metal |
|---|---|
| Feature Notes • FDA-approved materials for plastic components | |
Dimensions | |
| Diameter | 20 mm |
|---|---|
Dispensing | |
| Dispensing/Dosing Volume | 75 µl |
|---|---|
Overview | |
| Reference - Sub | Metering valve for inverted use |
|---|---|
| Description - Long MDI metering valve for inverted use, with 20 mm mounting cup. A collar for the complete emptying of the contents is employed. Suitable for HFA & CFC-propelled formulations and double stage filling over the internal gaskets. | |
| Packaging Type | Aerosols |
| Packaging Sub-Type | Aerosol Valves |
| Market - Major | Beauty, Personal Care. Health |
| Market - Segment | Cosmetics. Personal Care. Fragrances. Pharmaceuticals |
| Market - End Use | Nails. Hair. Hair Care. Female Fragrances. Male Fragrances. Illness Prevention. First Aid |
| Certifications | Laboratory, Testing, Certification. ISO Certifications. FDA Approved |
Characteristics | |
| Materials | Plastic. Metal |
|---|---|
| Feature Notes • FDA-approved materials for plastic components | |
Dimensions | |
| Diameter | 20 mm |
|---|---|
Dispensing | |
| Dispensing/Dosing Volume | 100 µl |
|---|---|




















